Which of the following would be more reactive than magnesium MG Chemistry. Which of the Following Would Be More Reactive Than Magnesium.
How To Determine The Reactivity Series Of Magnesium Aluminium Zinc And Copper By Electronegativity Or Something Else Quora
Answerpotassium is more reactive than Mg because both lie in the same group and the element potassium has more electropositivity than magnesium.
. The element which gets oxidised easily is more reactive. Which of the following will be more reactive than magnesium kCaBeAr Chemistry. Calcium is more reactive than magnesium because calcium atom is larger than magnesium atom and it has one more energy level.
How Does The Metal Reactivity Series Work Example Chemistry Education Chemistry Lessons Biology Facts. In the group 2 elements the reactivity of the metals increase as you move. Calcium is more reactive than magnesium because calcium atom is larger than magnesium atom and it has one more energy level.
Calcium because its atom is larger. K is more reactive. How many liters of fluorine gas at standard temperature and pressure will react with 235 grams of potassium metal.
This means that the electrostatic attraction between the valence electrons of calcium and the nucleus is weaker than in magnesium larger distance smaller force. In the Periodic Table Calcium is directly below Magnesium which means that a calcium atom is larger than a magnesium atom. We follow the reactivity series to say which one is more reactive than the other.
Correct answer to the question Guys. Oxidation is loss of electrons by an atom or a species. Lions 14K 7 months ago.
Mg has three outershells because of that its electrons are closer to the nucleus of atom and requires more energy in order to achieve full outer shell. Show all o. Anna 14 1 year ago.
Ca has four shells and its 2 electron in the outer shell is less attracted to the nucleus. How To Determine The Reactivity Series Of Magnesium Aluminium Zinc And Copper By Electronegativity Or Something Else Quora. Mg has three outershells because of that its electrons are closer to the nucleus of atom and requires more energy in order to achieve full outer shell.
You might be interested in. What would be more reactive than magnesium. Which of the following would be more reactive than magnesium.
Points brainliest which of the following elements is more reactive than the others. Ca has four shells and its 2 electron in the outer shell is less attracted to the nucleus. Calcium is more reactive.
See answer 1 Best Answer.
Which Is More Reactive Lithium Or Magnesium Quora
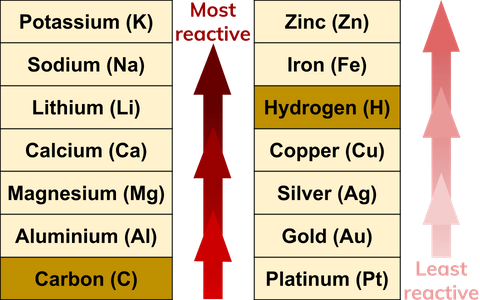
What Are Reactivity Series Definition From Seneca Learning
Which Is More Reactive Iron Or Hydrogen Quora
What Is The Reactivity Order Of Na K Li And Mg With Water Quora
0 Comments